Vanadium redox flow battery
The safest battery with almost unlimited service life
The battery from 1st Flow is based on vanadium redox flow technology. This technology stores energy in a liquid called the electrolyte.
The electrolyte, consisting of an acidified water solution with dissolved vanadium salts, stores energy by utilizing the four possible oxidation states of the vanadium cation.
During operation, electrolyte is pumped through electrochemical cells (stacks), where, depending on the direction of current flow, the charging or discharging reactions occur.
Vanadium ions are oxidized or reduced to different oxidation states inside the stacks. Since no reactions take place outside the stacks, unintentional self-discharge is eliminated.
Electronic control
The bidirectional inverter controls the storage and delivery of energy. The control system is self-regulating and compatible with commerical products.
Stack
If energy is needed, the electrolyte is pumped through the stacks.

Containment
Depending on where it is installed, the system is located inside a container, or housing made of steel or concrete. If the system is to be used indoors, the housing is not mandatory.
Tank
The electrolyte is stored in the tanks. The more electrolyte stored in the tanks, the larger the capacity of the battery. For additional safety against leaking, the system has a secondary containment unit.
Advantages
- Unlimited cycles as the electrolyte does not wear out
- No deep discharge or overcharging possible
- Sustainable. The electrolyte is fully recyclable and reuseable
- Safe. The electrolyte is non-flammable or explosive
- Easily scalable and compatible with energy infrastructures
Vanadium redox flow batterie’s capacity and power metrics can be tailored to meet the specific requirements of their intended use.




Technology
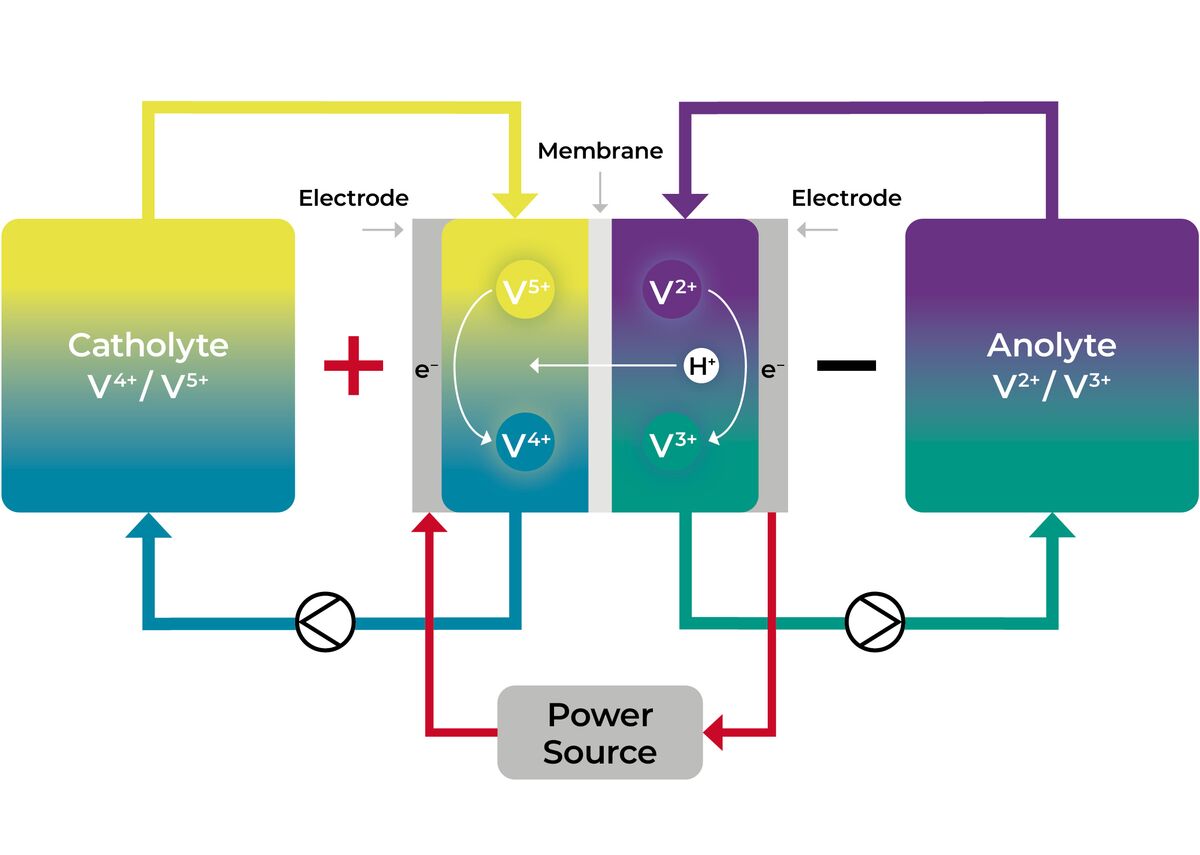
The redox flow process:
Innovative, safe, robust
The electrolyte is charged and discharged as it is pumped through the electrochemical cells of the stacks. The redox reactions depends on the direction of the current.
Charge
In one half-cell, vanadium is reduced from the 3+ oxidation state to the 2+ oxidation state.
Concurrently, vanadium is oxidized from the 4+ oxidation state to the 5+ oxidation state in the other half-cell.
Discharge
In one half-cell, vanadium is oxidized from the 2+ oxidation state to the 3+ oxidation state.
Concurrently, vanadium is reduced from the 5+ oxidation state to the 4+ oxidation state in the other half cell.
The redox reactions for both the charging and discharging processes must take place simultaneously in each electrochemical cell. If no electrolyte is pumped into the stack, no redox reactions take place. Complete, unintentional self-discharge is therefore technically not possible.
Charging and discharging are chemical reactions in a liquid medium. Apart from easy-to-replace pumps and valves, there are no mechanical components that requirement maintenance.
Therefore, there is no upper limit to the amount of charging and discharging cycles this battery technology can achieve, as often occurs with other storage technologies.
If the battery does need to be replaced at some point, the electrolyte can be fully recycled without issue. The vanadium can even be completely converted into new products.
Invest in your energy future. Store your renewable energy in a Redox Flow energy storage system from 1st Flow.

